3.b.2.b) Structure of the atom: What are electrons?
In the section on unstable Fundamental Particles with mass, we defined a new type of particles called wavons, which share the material nature of mass and the wave nature at several moments of their existence. Electrons were a specific case of wavons and if the atomic nucleus acquires or loses energy, the point of equilibrium that generated them before alters.
The mixed nature of electrons is independent of the dual property of matter, initially introduced by De Broglie in 1924, which refers to a different aspect. Furthermore, this duality of matter is different from the poorly named dual nature or behavior of light, as has been discussed in previous sections of this book.
In other words, electrons in the new structure of the atom in Global Mechanics do not magically appear and disappear, or come and go from other dimensions, as statements from the current Quantum Mechanics would seem to indicate.
Let us keep in mind that, besides the electron configuration, there are elements of the atom. The nucleus of protons and neutrons (particles with mass, or compressed matter) which possess most of the mass, as established by the Rutherford model in 1911 with the Geiger–Marsden experiment (also called the Gold foil experiment or the Rutherford experiment). Rutherford fixed the radius of the atom as approximately 10,000 times that of its nucleus.
The fundamental innovation of Global Mechanics, regarding the elements and structure of the atom, is the configuration of electrons because of the electromagnetic field, and as elements reducing the transversal tension of this field. The configuration is in contrast to Quantum Mechanics, which states that electrons in motion generate an electromagnetic field, although this is also true.
Perhaps it seems to be merely a philosophical change, but effect-cause is not the same as cause-effect, much less so is cause-cause, as a large part of the current Quantum Mechanics proposes.
Regardless, we hope that the new characteristics of the elements of the atom and its electron configuration will help to a more precise understanding of electrons, their origin, and their orbits.
The point of equilibrium where electrons exist is a dynamic equilibrium; what's more, however, the dynamics of movement of the electrons in the structure of the atom respond to different causes and display different behaviors.
Let us look at some of the additional characteristics of the structure of the atom and, in particular, its electron configuration. First, we shall examine electron motion within any orbit and subsequently, analyze both the reasons for which they change orbit and the way in which they do it.
The dynamic orbits of electrons
The most relevant change in the electron configuration of the new atomic model is, without a doubt, the shape and meaning of the orbits of the electrons.
The Rutherford atomic structure defines the electron orbits as circular and elliptical, the Bohr atomic theory presumes them to be circular, and the Sommerfeld model adds sublevels, rules out circular orbits and includes relativity. In the end, the current Schrödinger model changes the philosophy of atomic orbits and outlines areas of the probability of finding an electron in the spatial structure of the atom.
According to Global Mechanics, the electron configuration of the atomic structure also accepts the zones of spatial localization of negative charges around the nucleus –or electrons–, which belongs to the type of elementary particles, called wavons. Electrons have ellipsoid orbits that are variable despite being stable. As a result, the orbits represent the points through which the electrons move while they share the nature of mass; that is, as they are indeed wavons when they have the characteristic of coiled Global Aether of mass and not of an electromagnetic wave.
The electron orbits 
The orbits of electrons are dynamic, ellipsoid, not necessarily around the atomic nucleus, and they correspond to spatial points where the resulting force of electromagnetic tension –torsion–, and the tension of longitudinal curvature –or classic gravitational curvature–, is null. Alternatively, it is null due to the electron motion, the vibration of the nucleus of the atom and the half-fold or bend that form the electrons.
The movement of the wavon in orbit neutralizes –is a consequence of– the force of residual torsion or difference in the residual gravito-magnetic potential after the elastic energy of torsion neutralizes with the half-fold of the mass of the electron itself.
The orbits of the electron configuration are dynamic or have a cloud-like shape such as in the Schrödinger atom model of 1926, because of the vibration of the atomic nucleus. This vibration occurs because the distribution of elastic forces of torsion and tension of the longitudinal curvature is not uniform, nor can it have purely radial symmetry; like the force of gravity considered at greater distances than atomic distances.
Consequently, the orbits of the electron configuration in the new atom model will also be ellipsoid. The ellipsoid figure will not have to be on a single plane of space –instead, it will be a three-dimensional ellipsoid. Also, neither will the nucleus of the atom have to be located within the orbital cloud.
One could already see the Schrödinger structure of the atom; the zones of movement are not always entirely around the nucleus. Although the orbits of the electrons may be circular or elliptical, this will not always be the case. They will be ellipsoid.
Let us take a careful look at why the motion of electrons within an orbit responds to the electromagnetic energy not relaxed by the half-fold of which they formed.
The dance of the Wavons
The mass of the electron depends on the stored elastic energy. From a spatial perspective, the energy of the electrons will be equivalent to the neutralized elastic energy and will depend on the physical limit, for a half-fold, loop or curl of Global Aether create, and on its orbital speed.
However, the neutralization by the movement of the wavons in the structure of the atom takes place with each complete turn or revolution; that is, the only orbital frequencies allowed are those that can neutralize or relax the forces of torsion. At the same time, the speed of the electrons will neutralize these forces, since it depends on them. It is somewhat similar to when we want to touch something with the hand, and that something moves in the same direction and speed, our force or intention to touch or push it will become neutralized.
We do not know if it is just us today, or if it is tough to explain the elements of the new atomic structure or both, so we are going to try explaining it another way.
Electron configuration Magnetic field 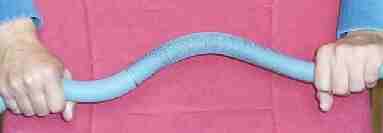
In the heyelogic figure, there is a pair of hands holding a polyurethane bar by the ends with torsion. If the hands move, like peddling a bicycle, in the same direction as the forces of torsion or twisting transversal tension, the tension at the ends of the bar held by the hands will not vary substantially. Nevertheless, if they move in the opposite direction, due to the elastic reaction in the bar, the tension in the hands will disappear once it reaches a certain rotation speed; the only thing that one can do is to let the two hands go along with the movement.
The tension produces an elastic force that tends to move the hands. If the hands move backward with the same speed that they would have had due to the effect of the elastic forces of torsion, the elastic forces will no longer be noticeable; that is, from this point on, outside of the hands, they do not exist. For future reference, we should name this mechanism of elastic relaxation in the structure of the atom. We like to call it the dance of the wavons.
The spatial points through which the electrons move in their dance are not the orbits around the nucleus, but rather they will move upon an axis of symmetry that can, in turn, be mobile, based on the result of the existing elastic forces at play.
Electronic configuration and Pauli exclusion principle
Electrons do not necessarily have to travel around the entire orbit –each of the two electrons in a single state could be moving back and forth in a particular section of the orbit. The movement would be due to the vibration in the atom from the elasticity forces in Global Aether when there are restrictions on atom’s movement in some way, such as when it forms part of a molecule. Recall that the electron exists in the equilibrium points of elastic forces and depends on the location of the nucleus of the atom concerning its surroundings.
The following example of a hard plastic ball and its resistance to deformation gives an intuitive idea of the Pauli exclusion principle.
-
Simple Physics experiment
If one were to kick this ball, nothing would happen; however, if it was a particularly hard kick, it might produce a dent in its surface in the shape of a largish slice of orange.
Now, if one were to continue giving little kicks all over the ball, harder and harder each time, one would find that the next orange-slice dent would appear just at the antipode of the first, with the same orientation. Afterward, two more will fit in the perpendicular plane. Finally, four more in the intermediate spaces.
Global atom elasticity
Global aether force equilibrium
Of course, everything would depend on the elasticity of the plastic. If we could define these forces mathematically, we could prove and generalize that under certain conditions, one would always obtain the same result.
Then, the Pauli exclusion principle would cease to be a principle and become a physical law based on a mathematical theorem representing particular conditions.
An atom with a higher number of protons will have a more significant transversal tension difference between the filaments in the atomic nucleus and the outside of the atom. Therefore, more electrons will create further away if the inner layers are adjusted and relaxed with electrons. We must bear in mind that the tension of longitudinal curvature (different from the transversal one) decreases with distance to the atomic nucleus.
On the other hand, some electrons may form earlier in particular locations of higher levels with more tension than the inner layers due to the spatial geometry of the elasticity.
For a formal analysis see the Wikipedia page on electronic configuration. ** There are exceptions and many practical rules, such as the s + d + n rule and the Aufbau principle.
-
-
Electron spin and orbital angular momentum
The confirmation of the existence of electron spin came about thanks to the Stern-Gerlach experiment and the so-called fine structure of the Hydrogen spectrum.
The electronic configuration given previously is coherent with the Pauli exclusion principle, the existence of spin or intrinsic angular momentum of electrons and the Spin-orbit interaction –such as the fine structure of Hydrogen. See the HyperPhysics ** page for more details on electronic spin.
The sign of the Spin seems to be simply due to whether the orbital angular momentum is in the same direction as the magnetic moment of the electron due to spin itself, or in the opposite one. Consequently, the positive or negative values of spin depending on the Spin-orbit interaction.
Vibration of the atom
Spin changes when electron flips
Topological insulators are an example of the relationship between Spin and linear momentum –in these objects, a Spin-impulse block takes place.
From a different perspective, the origin of spin comes almost certainly from the energetic barrier of stability in the creation of electrons, which is undoubtedly part of the intrinsic nature of these particles and possibly linked to the creation of individual neutrinos.
-
Tunnel effect or leap between electron orbits
If the nucleus of the atom acquires energy by absorbing a photon, it will change the structure of the generated gravito-magnetic field, as well as the points of equilibrium where the electrons can exist and move. Hence, at times when the imbalance is higher than the energy barrier of stability of the electrons, the mass of the electrons will vanish into electromagnetic energy, until the half-fold, loops or curls that make up the electron mass will once again create, reaching a new point of orbital equilibrium.
Therefore, it is not possible to follow the electron motion between orbits, and Modern Physics talks about electrons as leaping between orbits in the structure of the atom and about the movement of electron clouds.
This mixed nature of the electrons is also the basis of a possible explanation for the tunnel effect and the Young experiment, or double slit experiment carried out with electrons.
Free electrons and molecular bonds
Electrons can also create between different atoms, forming covalent, ionic or metallic bonds.
They equally move like stable subatomic particles with mass like a slipknot in the classical vacuum or reticular structure of matter or Global Aether.In these cases, they are free electrons because they can leave the space of the atom or molecule. From Global Mechanics, what has happened is either that the variations of the energy of the atomic nucleus provoke changes in the spatial localization of the relaxation points of the transversal torsion of Global Aether, or that the relaxation is not necessary anymore.
Likewise, the electron motion in exterior space, or classical vacuum, shows that they have certain stability, and therefore there must be an energy barrier –minimum of energy– for which the electron breaks up –decays– into photons. Also, it is possible that the more kinetic energy they possess, the more stable they will be.
The stability of the electron will affect the configuration of the orbitals in the atom since it will delay the elastic adjustments of the whole atom. This characteristic of the electrons contributes to a higher spatial margin of the spheroid shape of their orbits.
Simple physics experiment.
In the example of the slipknot with a hair, one can see how quickly the knot slides.
Now, for the case of electrons, let us think that the slipknot is a half-knot, which created with a bend in the straw of a refreshing drink.
Intuitively, we can see how this bend will only occur above the minimum energy of transversal turn on said straw. Otherwise, the straw will preserve its cylindrical shape.
Crack of Global Aether
Stability energy barrier
The electrons –or the bend in the plastic straw in our example– will have the same resistance or energy barrier to disappear than it had to appear.
We have just discovered another one of the possible characteristics of the filaments of Global Aether, that is, they will have a tubular nature, though it will not be utterly homogeneous due to the vertices of the cubic cells of the three-dimensional net.
As we know from the photoelectric effect, the electron will have higher speed and greater kinetic energy the more significant the energy of the photon absorbed by the atom is, always above a necessary minimum of energy. Without said minimum energy, no electron will outflow, no matter how much we increase the radiation intensity.
A recent experiment in the limits of the photoelectric effect carried out by German scientists show that an absorbed photon could produce the expulsion of more than one electron; in other words, it seems that in this case, the nucleus of the atom –and not the electron– absorbs the photon.
